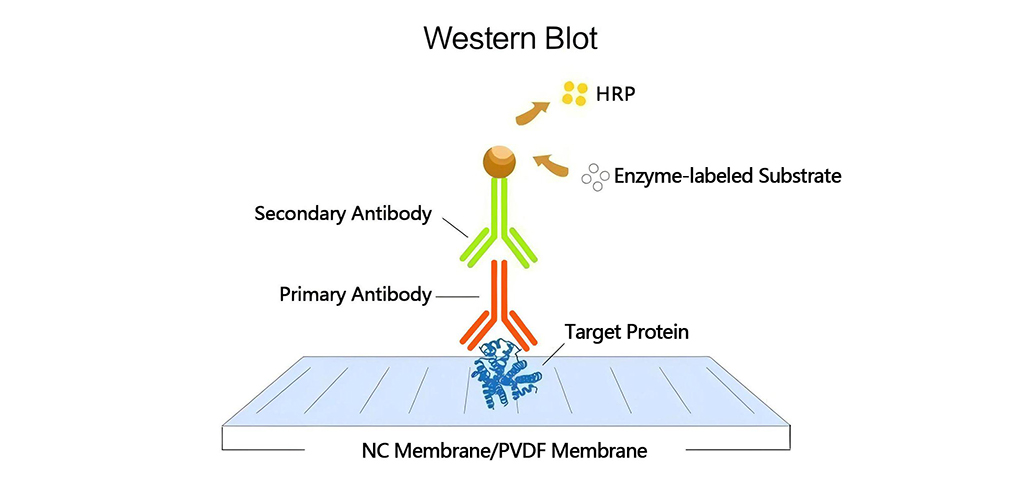
In conventional immunoblotting workflows, protein samples are first separated by SDS-PAGE and then transferred onto a membrane under an electric field. After blocking, the membrane is incubated with a primary antibody targeting the antigen of interest. Following washing, the membrane is incubated with an enzyme-conjugated secondary antibody targeting the primary antibody. The enzymatic activity, such as alkaline phosphatase (AP) or horseradish peroxidase (HRP), is essential for signal generation. Finally, the membrane is washed again and incubated with the appropriate enzyme substrate to produce a detectable signal.
The most commonly used HRP substrates are based on luminol, which generates chemiluminescent signals. The principle of chemiluminescence involves the release of energy in the form of light during a chemical reaction. In the presence of HRP and a peroxide buffer, luminol is oxidized and forms an excited-state product, emitting light as it decays to the ground state. Since light is emitted only during the enzyme-substrate reaction, signal output ceases once the substrate near the enzyme is depleted. In contrast, precipitates generated by chromogenic substrates (e.g., DAB) remain visible on the membrane even after the reaction is terminated.
Under optimal conditions for all immunoblotting factors, chemiluminescent signals can persist for 6–24 hours, depending on the specific substrate used and the enzyme-to-substrate ratio in the system. Rapid signal decay may lead to bias, low sensitivity, or failed signal capture. Prolonged signal duration minimizes variability caused by transfer efficiency, substrate batch differences, and other factors. During the oxidation reaction of HRP and luminol, generated free radicals can bind to HRP, rendering the enzyme unable to interact with the substrate. A high concentration of HRP in the system increases the likelihood of HRP inactivation due to the generation of excessive free radicals. Free radicals can also damage antigens, antibodies, and the membrane, compromising the effectiveness of re-probing.
Strategies to Optimize Signal Intensity and Duration
-Blotting Membrane
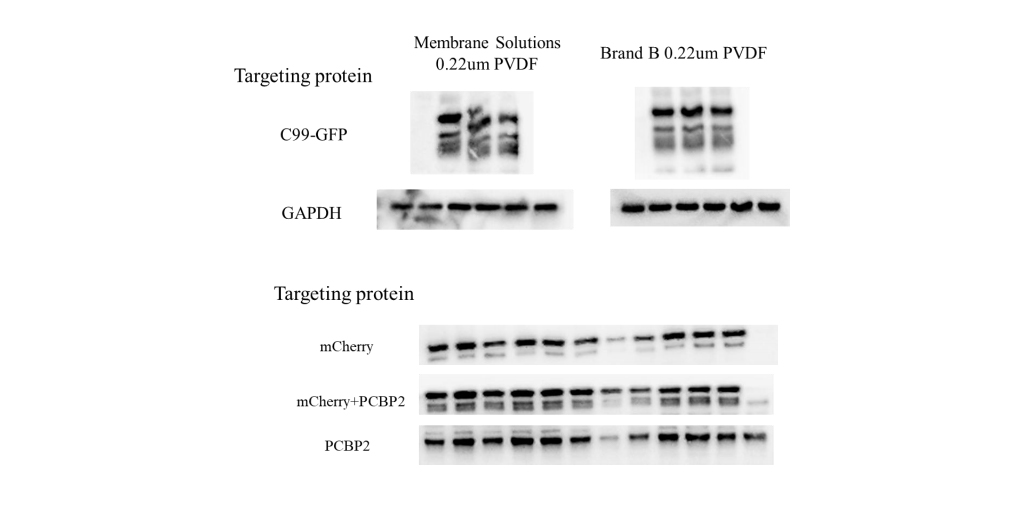
The membrane composition generally does not affect HRP-luminol interactions or subsequent signal generation. However, nitrocellulose (NC) and PVDF membranes differ in protein-binding properties. PVDF membranes typically exhibit stronger protein-binding capacity, higher tensile strength, and superior handling characteristics. However, their higher hydrophobicity can make wetting difficult and sometimes result in elevated background signals. For optimal results, test the membrane empirically before using precious samples or antibodies. Given potential batch-to-batch variability, evaluating new membrane batches beforehand is advisable.
Protein Transfer
Transfer efficiency varies significantly among proteins due to differences in gel migration behavior and membrane-binding tendencies under varying conditions. Transfer efficiency depends on factors such as gel composition, gel-membrane contact, electrode placement, transfer duration, protein size and composition, field strength, buffer system, and the presence of detergents. Most proteins achieve optimal transfer under low ionic strength buffers and low-current conditions. However, rapid transfers require high ionic strength and can be performed at high currents. Post-transfer, membranes can be stained with immunoblot-compatible or reversible dyes to assess transfer efficiency.
Blocking Buffer
A wide range of blocking reagents is available for immunoblotting. Since no single blocking reagent suits all systems, selection must be based on experimental outcomes. The ideal blocking buffer maximizes the signal-to-noise ratio without reacting with the system’s antibodies or targets. For example, when using an avidin/biotin system, 5% skim milk as a blocking reagent can cause high background due to its high endogenous biotin content, which binds to avidin. Additionally, milk contains phosphatases that may dephosphorylate protein samples, affecting phosphoprotein detection. Changing substrates, antibodies, or targets may reduce signal or increase background if the blocking buffer is suboptimal for the new system.
Some systems benefit from adding surfactants to the blocking buffer. Surfactants can prevent nonspecific binding of blocking reagents to targets, thereby minimizing background. However, excessive surfactant can impair blocking efficacy. Typically, a final detergent concentration of 0.05% is used, though not all systems require surfactant optimization.
Antibodies
Protein immunoblotting typically employs primary antibodies that recognize specific proteins or epitopes within complex protein mixtures. The choice of primary antibody depends on the target antigen and available antibodies. Note that not all primary antibodies are suitable for immunoblotting; only those validated for this application should be used. Detection usually involves labeled secondary antibodies (indirect detection). A variety of labeled secondary antibodies are available for immunoblotting, with selection based on the primary antibody’s host species or tags (e.g., biotin, 6xHis, HA, etc.).
The affinity of the primary antibody for the antigen is critical, and the concentrations of both primary and secondary antibodies profoundly influence signal generation. Overly strong signals on the membrane may result from excessive concentrations of the primary or secondary antibody (or both). Generally, minimizing the primary antibody concentration is beneficial, as it promotes target-specific binding and reduces background. If signals are weak due to high background or low antibody binding, enhancer products can be used as a pretreatment to boost immunoblotting signals. For example, Western Blot Enhancer (Cat. No. W2500) can improve target protein detection.
If insufficient signal is captured on the membrane, stripping all detection reagents from the membrane and re-probing with the same primary antibody at a different concentration or a different primary antibody at the same concentration is recommended. This often saves precious samples and time. However, incomplete stripping may leave active HRP on the membrane, generating interference signals. To check for residual active HRP, reapply the substrate and detect the signal. If large amounts of inactive HRP remain, they may also inhibit primary antibody binding during re-probing. Stripping and re-probing is an efficient method to gather system-specific information while conserving valuable samples.
Ucallm ECL Technical Parameters Comparison
Product information | W2501 Ultrasensitive ECL chemiluminescent liquid | W2502 Ultra-sensitive ECL chemiluminescent liquid |
Applicable samples | First choice for daily experiments, low abundance of target protein, limited samples | The target protein is extremely low in abundance, the sample is extremely precious or the antibody is limited |
Performance advantages | High cost performance, long-lasting signal | The highest sensitivity, less optimization operation |
Detection sensitivity | Picogram to low femtogram level | Low femtogram level |
Signal duration | Up to 12 hours after optimization | 6 hours |
Storage stability | 2 years at 4℃ | One year at 4℃, half a year at room temperature |
Note: Recommended antibody dilution concentrations are based on a stock concentration of 1 mg/ml.





Leave a Comment
Your email address will not be published. Required fields are marked *