Protein Sample Preparation
-Sample Quality Control
- Protein Integrity: Verify protein concentration (Bradford/BCA assay) and ensure complete denaturation (boiling in Laemmli buffer)
- pH Optimization: Maintain sample pH 7.0-8.0 for optimal electrophoretic mobility
- Degradation Prevention: Include protease inhibitors (e.g., PMSF, cocktail tablets) during extraction
- Cell Requirements: 5×10⁶ cells typically yield sufficient protein for standard WB analysis
Special Considerations by Molecular Weight
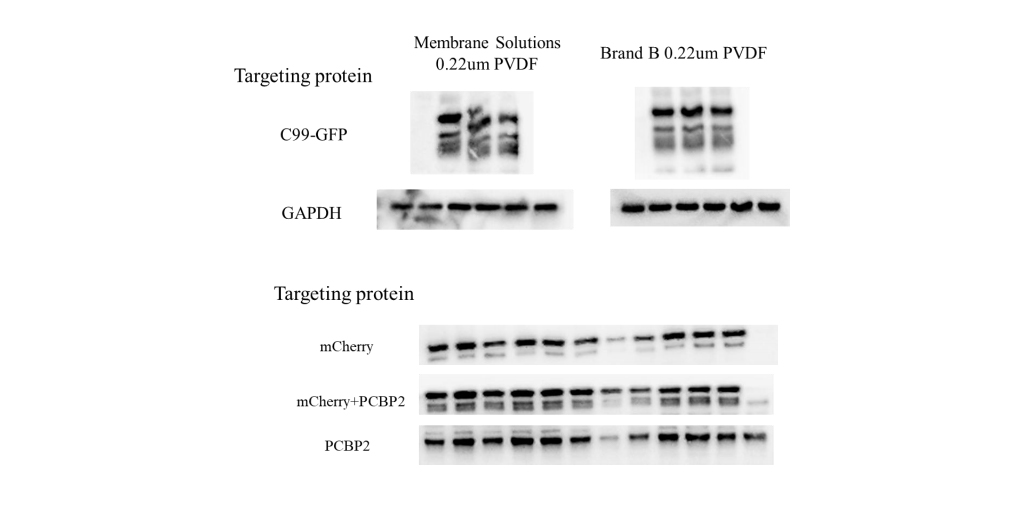
- Membrane selection: 0.22 μm PVDF or nitrocellulose
- Electrophoresis: Tricine-SDS-PAGE system recommended
- Transfer protocol: Reduce transfer time or use double-membrane technique
-For Large Proteins (≥200 kDa):
- Gel composition: ≤7% acrylamide separation gel
- Transfer conditions: Extended duration (overnight possible)
- Reference markers: Essential for proper band identification
- Methanol adjustment: Reduce to 5-10% in transfer buffer
Gel Preparation Protocol
-Critical Parameters
- pH Precision:
- Separation gel: 8.8 ± 1
- Stacking gel: 6.8 ± 1
- Polymerization Control:
- Use fresh APS/TEMED solutions
- Optimal polymerization time: 30-45 min (RT)
-Troubleshooting Guide
Issue | Solution |
Gel surface curvature | 1. Apply water/isopropanol gently along plate edge |
2. Ensure perfectly horizontal surface | |
3. Use 100% isopropanol for sealing | |
Incomplete polymerization | 1. Verify APS freshness (<2 weeks at 4°C) |
2. Adjust TEMED concentration (0.1% final) |
Sample Loading Techniques
-Best Practices
- Loading Method: Use gel-loading pipette tips for bottom deposition
- Volume Management:
- Standard: 20-30 μg protein/lane (10-well comb)
- Overload solutions:
- Sample concentration (ultrafiltration)
- Increased gel thickness (1.5mm)
-Electrophoresis Conditions
- Optimal Parameters
- Buffer System: Fresh 1×Tris-Glycine-SDS (pH 8.3)
- Voltage Protocol:
- Stacking: 80V constant
- Separation: 100-120V constant
- Temperature Control: Maintain ≤25°C (cooling circulator recommended)
Membrane Transfer Protocol
-Selection Criteria
Membrane Type | Optimal Application |
0.45 μm NC | Routine detection (>30 kDa) |
0.2 μm PVDF | Small proteins (<20 kDa) |
PSQ | Specialized applications |
-Transfer Conditions
- Standard Protocol: 100V, 60-90 min (4°C)
- Large Proteins: 30V overnight
- Buffer Composition: 25 mM Tris, 192 mM glycine, 20% methanol
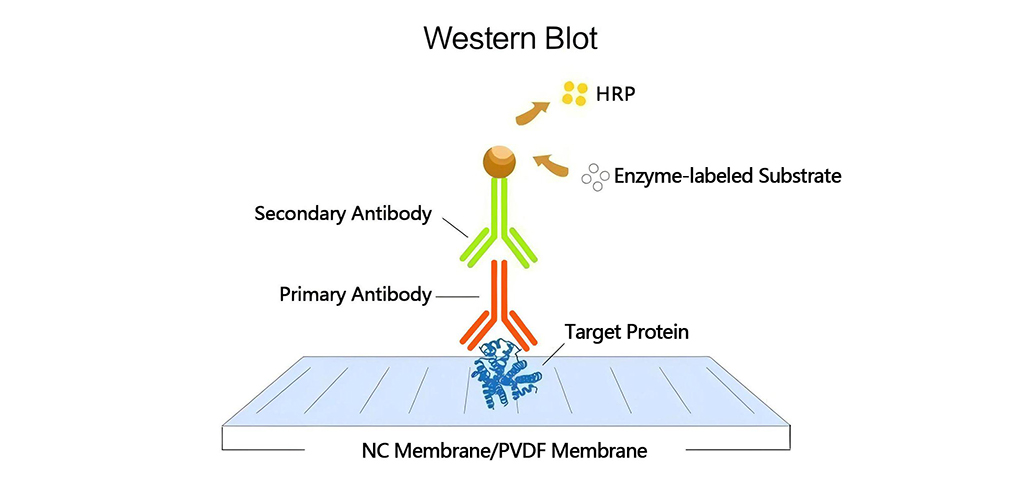
Immunodetection Protocol
-Blocking Strategies
Blocking Agent | Recommended Use |
5% Non-fat dry milk | General purpose |
5% BSA | Phosphoprotein detection |
Commercial blockers | High sensitivity applications |
-Incubation Conditions:
- Time: 1h (RT) to overnight (4°C)
- Buffer: TBST (0.1% Tween-20)
-Antibody Considerations
- Primary Antibodies:
- Species: Prefer rabbit/mouse monoclonal
- Validation: Verify linear epitope recognition for WB
- Storage: Aliquot at -20°C; avoid freeze-thaw cycles
- Secondary Antibodies:
- Conjugates: HRP/fluorescence (choose based on detection system)
- Incubation: 1h RT (protected from light)
-Detection Methods
- Chemiluminescent Detection
- Substrate Selection: Enhanced chemiluminescence (ECL) for sensitivity
- Exposure Time:30s-5min (optimize for signal-to-noise)
- Antibody Reuse Guidelines
- Primary Antibody:Maximum 3 reuses (4°C storage with 0.02% sodium azide)
- Secondary Antibody: Single use recommended
Technical Comparison: WB vs IHC Antibodies
Characteristic | Western Blot | Immunohistochemistry |
Epitope Recognition | Linear only | Linear + conformational |
Protein State | Denatured | Native |
Antibody Suitability | Requires validation | Broader applicability |
For optimal results, we recommend our premium Western Blot Starter Kit containing optimized buffers, membranes, and detection reagents. Technical support available for protocol customization.





Leave a Comment
Your email address will not be published. Required fields are marked *