When analyzing Western blot results, researchers often encounter unexpected bands that can compromise experimental outcomes. These non-specific bands can arise from various sources, including antibody issues, sample preparation problems, and buffer composition. Understanding the causes of these unwanted bands is crucial for troubleshooting and obtaining reliable, reproducible Western blot results.
We will examine the various factors that contribute to non-specific bands in Western blots, with particular attention to elution buffer issues and practical solutions to overcome these challenges. By identifying the root causes of these issues, you can optimize your Western blot protocol to achieve more accurate and reliable results.
Key Takeaways
- Non-specific bands can arise from various sources, including antibody issues and buffer composition.
- Understanding the causes of non-specific bands is crucial for troubleshooting Western blot results.
- Elution buffer issues can significantly impact Western blot outcomes.
- Practical solutions can be implemented to overcome non-specific band challenges.
- Optimizing Western blot protocols can lead to more accurate and reliable results.
Understanding Non-Specific Bands in Western Blot
Non-specific bands can obscure the results of Western blot analysis, making data interpretation difficult. When performing a Western blot, non-specific bands are unexpected protein bands that appear due to antibodies binding to proteins other than the intended target.
What are non-specific bands?
These extraneous bands can appear above, below, or at similar molecular weights to the target protein, creating confusion in data interpretation.
Impact on experimental results
The presence of non-specific bands can lead to false positives, misidentification of proteins, and potentially invalid experimental conclusions. You must distinguish between true protein signals and artifacts.
Common locations of non-specific bands
Non-specific bands commonly appear due to cross-reactivity, sample contamination, or technical issues during the Western blot procedure.
Understanding the causes is the first step toward implementing effective solutions.
Antibody-Related Causes of Non-Specific Bands
Antibody-related factors are a common cause of non-specific bands observed in Western Blot analyses. The specificity and characteristics of the antibodies used play a crucial role in the accuracy of the results.
Polyclonal vs. Monoclonal Antibodies
The choice between polyclonal and monoclonal antibodies can significantly impact the specificity of Western Blot results. Polyclonal antibodies, by recognizing multiple epitopes on the target protein, can increase the likelihood of cross-reactivity with structurally similar proteins, resulting in non-specific bands.
- Polyclonal antibodies recognize multiple epitopes, potentially leading to cross-reactivity.
- Monoclonal antibodies offer higher specificity but may still exhibit cross-reactivity with proteins containing similar epitopes.
Primary Antibody Concentration Issues
Optimizing the concentration of the primary antibody is critical. Excessive concentrations can lead to non-specific binding due to low-affinity interactions with non-target proteins.
- Too high concentrations cause background noise and non-specific bands.
- Too dilute solutions may result in weak specific signals.
Antibody Cross-Reactivity
Cross-reactivity can occur due to shared epitopes between different proteins or post-translational modifications. Using pre-adsorbed antibodies or validating antibodies with appropriate controls can mitigate this issue.
- Shared epitopes or post-translational modifications can cause cross-reactivity.
- Pre-adsorbed antibodies or validation controls can reduce non-specific binding.
Sample Preparation Factors Leading to Non-Specific Bands
Non-specific bands in Western Blot can often be attributed to issues during sample preparation. Several factors can contribute to this problem, including protein degradation, protein multimers and aggregates, and sample overloading.
Protein Degradation
Protein degradation during sample preparation can generate fragments that retain antibody-binding epitopes, resulting in multiple bands at lower molecular weights than the intact protein. To prevent this, it’s essential to use protease inhibitors, maintain samples at cold temperatures, and minimize freeze-thaw cycles.
Protein Multimers and Aggregates
Protein multimers and aggregates can form when proteins associate through covalent or non-covalent interactions, creating bands at higher molecular weights than expected. Insufficient sample denaturation or reduction can preserve protein complexes and disulfide bonds, leading to unexpected high molecular weight bands on Western blots.
Sample Overloading
Sample overloading can overwhelm the separation capacity of the gel, causing smearing, distorted bands, and non-specific background signals. To avoid this, optimal protein loading is crucial. For cell lysates, aim for 20-30 μg per well, and for purified proteins, 10-100 ng is typically sufficient.
| Sample Type | Optimal Protein Loading |
|---|---|
| Cell Lysates | 20-30 μg |
| Purified Proteins | 10-100 ng |
By optimizing sample preparation and being aware of these potential issues, you can reduce the occurrence of non-specific bands and improve the accuracy of your Western Blot results.
Cell Line and Protein Characteristics
Understanding the characteristics of your cell line and protein is vital for accurate Western blot analysis and minimizing non-specific bands. The inherent properties of your cell line and the specific protein you’re analyzing can significantly influence the outcome of your Western blot.
High Passage Cell Lines
High passage cell lines can accumulate genetic and epigenetic changes, leading to altered protein expression profiles. Using less-frequently or non-passaged cells can help mitigate this issue, reducing the likelihood of unexpected bands in your Western blot.
Protein Subtypes and Splice Variants
Some proteins have various subtypes or splice variants that share similar epitopes, causing antibodies to recognize multiple protein forms. This can result in additional bands on your Western blot, which may be mistakenly identified as non-specific.
Basal Expression in Control Samples
Basal expression of proteins in control samples can sometimes be misinterpreted as non-specific bands. It’s essential to understand the normal expression pattern of your protein of interest to accurately interpret your Western blot results.
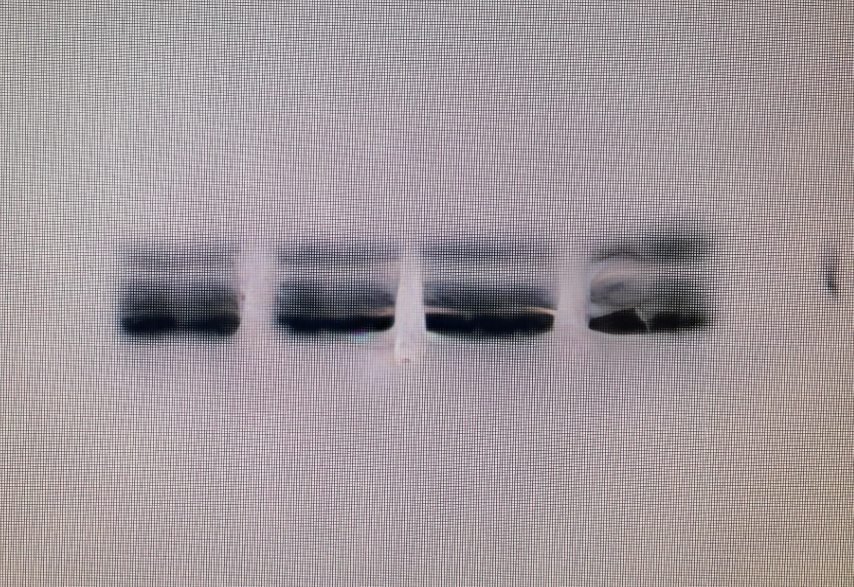
Non-specific bands in WB experiment
Buffer and Blocking Problems Causing Non-Specific Bands
Optimizing buffer and blocking conditions is essential for reducing non-specific binding in Western Blot. When buffers and blocking agents are not optimized, it can lead to non-specific bands, compromising the accuracy of the results.
Insufficient Blocking
Insufficient blocking is a primary cause of non-specific bands as it leaves membrane sites available for non-specific antibody binding. To address this, you can try boosting the concentration of your blocking reagent, such as increasing it from 2-5% BSA, or increasing the blocking incubation times.
- Optimizing blocking buffer composition, typically 3-5% BSA or non-fat dry milk, can significantly reduce background and non-specific signals.
- Preparing the primary antibody in the blocking buffer rather than antibody diluent alone can further reduce non-specific interactions.
SDS Contamination
SDS contamination in buffers can denature antibodies and alter protein conformation, leading to non-specific binding and unexpected bands. Thorough washing after transfer removes residual SDS from electrophoresis, preventing interference with antibody-antigen interactions.
Elution Buffer Composition Issues
Elution buffer composition issues, particularly improper pH or ionic strength, can affect protein-antibody interactions and contribute to non-specific binding. Adding detergents like Tween-20 (0.05-0.1%) to blocking and washing buffers helps reduce hydrophobic interactions that contribute to non-specific binding.
By addressing these buffer and blocking issues, you can significantly improve the specificity and accuracy of your Western Blot results.
Washing and Detection Protocol Issues
Optimizing washing and detection protocols is crucial for minimizing non-specific bands in Western blots. When washing protocols are inadequate, residual reagents can interfere with detection antibodies, leading to high background and non-specific binding.
Inadequate Washing Steps
Insufficient washing between steps is a major contributor to non-specific bands. To address this, ensure that enough washing buffer is used to cover the blot, and implement gentle agitation. Increasing the number of washes, typically to 4-5 times for 5 minutes each, can also help remove unbound antibodies.
Detergent Concentration
The concentration of detergent in washing buffers is critical. Too little detergent fails to remove non-specific binding, while excessive amounts can strip specific antibody interactions. Optimizing Tween-20 concentration to 0.05-0.1% helps balance effective removal of non-specific binding while preserving specific antibody-antigen interactions.
Incubation Time Optimization
Incubation times for blocking, primary antibody, and secondary antibody steps significantly impact specificity. Too short incubation times lead to incomplete reactions, while excessive incubation can increase non-specific binding. Temperature control during incubation steps also affects binding kinetics.
| Protocol Component | Optimization Strategy | Benefit |
|---|---|---|
| Washing Buffer | Increase volume, gentle agitation, multiple washes | Reduces non-specific bands |
| Detergent Concentration | Optimize Tween-20 to 0.05-0.1% | Balances removal of non-specific binding and preserves specific interactions |
| Incubation Time | Optimize incubation times for blocking and antibody steps | Improves specificity and reduces non-specific binding |
Step-by-Step Troubleshooting Guide for Non-Specific Bands
We guide you through a step-by-step troubleshooting process to effectively minimize non-specific bands in your Western Blot experiments. Nine out of ten times, this systematic approach is enough to fix most problems.
Systematic Approach to Identify the Cause
To troubleshoot non-specific bands, start by isolating variables. Begin with the most common causes such as antibody concentration and blocking efficiency. We recommend starting with fresh reagents and buffers to ensure there’s no contamination.
- Implement proper controls, including no-primary antibody controls, isotype controls, and positive/negative sample controls.
- Titrate primary antibody concentration to find the optimal dilution.
- Modify blocking protocols by testing different blocking agents and increasing blocking time.
Controls to Implement
Proper controls are crucial to distinguish between specific and non-specific signals. Use controls to validate your experimental setup and ensure that the observed bands are specific to your protein of interest.
Protocol Modifications
Adjusting your protocol can significantly reduce non-specific bands. Consider the following modifications:
- Adjust washing stringency by increasing wash buffer volume, duration, and number of wash steps.
- Test different buffer compositions and detergent concentrations to optimize specificity.
- Consider pre-adsorption of primary antibodies with cell/tissue lysates to remove non-specifically binding antibodies.
- Document all protocol modifications systematically to identify effective changes.

Reasons for nonspecific bands in WB experiments
Conclusion
As we conclude our series on Western Blot troubleshooting, it’s clear that addressing non-specific bands is multifaceted. We have identified that issues arise from various factors, including antibody properties, sample preparation, and buffer composition. To eliminate these bands, optimizing antibody concentration, blocking conditions, and washing protocols is crucial.
By understanding protein characteristics and implementing proper controls, you can significantly improve Western Blot reproducibility and reliability. For more troubleshooting tips and advanced techniques, continue exploring our blog for valuable insights.
References and further readings:
1.Grant, M. K. O., Shapiro, S. L., & Liu, P. (2019). A cautionary tale: Endogenous biotinylated proteins and protein A cause antibody-independent artefacts in Western blot. Biological Procedures Online, 21, 8.
https://link.springer.com/article/10.1186/s12575-019-0095-z2.Sousa, M. M. L., Steen, K. W., Hagen, L., & Slupphaug, G. (2011). Antibody cross-linking and target elution protocols significantly modulate signal-to-noise ratio in 2D-PAGE Western blot. Proteome Science, 9(45).
https://link.springer.com/article/10.1186/1477-5956-9-453.Blancher, C., & Cormick, R. M. (2012). SDS-PAGE and Western Blotting Techniques. In Metastasis Research Protocols. Springer Protocols Handbooks.
https://link.springer.com/protocol/10.1007/978-1-61779-854-2_6
FAQ
What are the primary causes of non-specific binding in Western Blot?
The primary causes include issues with the primary antibody, such as cross-reactivity or high concentration, insufficient blocking, and problems with sample preparation, like protein degradation or overloading.
How can I minimize non-specific binding due to the primary antibody?
To minimize non-specific binding, we recommend optimizing the primary antibody concentration, using a suitable blocking buffer, and selecting antibodies with high specificity for the target protein.
What role does the blocking buffer play in reducing non-specific binding?
The blocking buffer is crucial in reducing non-specific binding by saturating non-specific binding sites on the membrane, thereby preventing the primary antibody from binding to these sites.
How does sample overloading affect Western Blot results?
Sample overloading can lead to non-specific binding and reduced specificity, as excessive protein can overwhelm the membrane’s binding capacity, causing the primary antibody to bind non-specifically.
What are some common issues with elution buffer composition that can cause non-specific binding?
Issues with elution buffer composition, such as high salt concentrations or the presence of detergents like SDS, can cause non-specific binding by altering the protein’s structure or interacting with the membrane.
How can I troubleshoot non-specific binding in my Western Blot?
To troubleshoot, we suggest a systematic approach, starting with optimizing the primary antibody concentration, adjusting the blocking buffer, and modifying the washing and detection protocols as needed.
What is the impact of inadequate washing steps on Western Blot results?
Inadequate washing steps can lead to non-specific binding and background noise, as residual reagents can interact with the membrane or antibodies, compromising the specificity of the assay.
How can I optimize the incubation time for my Western Blot?
Optimizing the incubation time involves balancing the need for sufficient binding with the risk of non-specific binding; we recommend starting with recommended incubation times and adjusting as needed based on the results.
Leo Bios
Hello, I’m Leo Bios. As an assistant lecturer, I teach cellular and
molecular biology to undergraduates at a regional US Midwest university. I started as a research tech in
a biotech startup over a decade ago, working on molecular diagnostic tools. This practical experience
fuels my teaching and writing, keeping me engaged in biology’s evolution.





Leave a Comment
Your email address will not be published. Required fields are marked *