The viewpoint that Western blot analysis represents the gold standard for detecting cellular signaling events is absolutely correct. This is particularly true when examining restricted smad protein phosphorylation patterns in research applications.
TGF-β superfamily ligands control critical cellular processes through serine-threonine receptor kinases. These signaling pathways activate specific type I receptors that target distinct transcription factors. The canonical signaling mechanism involves precise modifications in SXS motifs for transcriptional regulation.
We provide comprehensive detection methodologies for studying these evolutionarily conserved signaling molecules. Our expertise demonstrates how transforming growth factor-β superfamily members signal through heteromeric receptor complexes. You will gain understanding of accurate detection methods essential for research professionals.
These detection techniques are crucial for studying diverse developmental processes and disease pathogenesis. The importance of precise analytical methods cannot be overstated in modern cellular biology research.
Key Takeaways
- Western blot analysis provides precise detection of cellular signaling modifications
- TGF-β superfamily ligands activate serine-threonine receptor kinases for transcriptional control
- Canonical signaling pathways target specific SXS motifs in transcription factors
- Accurate detection methodologies are essential for research applications
- These techniques help study developmental processes and disease mechanisms
- Heteromeric receptor complexes mediate transforming growth factor-β signaling
Understanding Restricted Smad Proteins
Restricted Smad proteins operate as key regulatory elements within complex signaling networks that control cellular behavior. These evolutionarily conserved transcription factors play essential roles in mediating responses to extracellular signals. We provide comprehensive analysis of their fundamental architecture and functional mechanisms.
The SMAD signaling pathway represents one of the most critical cellular communication systems in biological organisms. Eight distinct Smad family members work together to transmit signals from cell surface receptors to the nucleus. Each member contributes unique regulatory functions that maintain cellular homeostasis.
Role of Smad Proteins in Cellular Processes
Smad proteins function as molecular messengers that translate extracellular signals into specific gene expression changes. They regulate diverse cellular processes including proliferation, differentiation, and apoptosis. These proteins respond to transforming growth factor-beta superfamily ligands.
We observe that Smad proteins control developmental programs across multiple species. Their regulatory influence extends to tissue repair, immune responses, and metabolic processes. Cellular signaling cascades depend on precise Smad protein interactions for proper function.
The proteins maintain evolutionary conservation from simple organisms like Drosophila and Caenorhabditis elegans to complex mammals. This conservation demonstrates their fundamental importance in biological systems. Their regulatory networks have remained stable throughout evolutionary history.
Types of Smad Proteins: R-Smad, Co-Smad, and I-Smad
Three distinct categories of Smad proteins execute specialized functions within signaling networks. Receptor-regulated Smads (R-Smads) directly interact with activated receptor complexes. These proteins include Smad1, Smad2, Smad3, Smad5, and Smad8.
Common Smads (Co-Smads) serve as universal partners for R-Smads. Smad4 represents the primary Co-Smad that facilitates nuclear translocation. This protein enables transcriptional activation of target genes.
Inhibitory Smads (I-Smads) function as negative regulators within the system. Smad6 and Smad7 interfere with Smad-receptor interactions to prevent signal transmission. These proteins lack MH1 domains, distinguishing them structurally from other family members.
Importance of Phosphorylation in Smad Function
Phosphorylation events control Smad protein activation and cellular localization. Receptor-mediated phosphorylation occurs at specific serine residues within the MH2 domain. This modification triggers conformational changes that enable protein-protein interactions.
The structural organization features globular MH1 and MH2 domains connected by flexible linker regions. MH1 domains provide DNA-binding capability, while MH2 domains facilitate protein interactions. Linker regions contain regulatory phosphorylation sites that modulate activity.
We demonstrate how phosphorylation status determines Smad protein function within the SMAD signaling pathway. Different kinases target specific residues to create diverse regulatory outcomes. This modification system enables precise control of cellular responses to environmental signals.
Mechanism of Smad Protein Phosphorylation
TGF-beta induced phosphorylation mechanisms reveal sophisticated regulatory networks controlling Smad protein activity. We understand that these molecular processes form the foundation of cellular communication systems. The phosphorylation cascade begins when transforming growth factor-β superfamily members bind to their specific receptor complexes.
The receptor complex consists of two type II and two type I receptors working together. Type II receptors maintain constitutive kinase activity, making them ready to initiate signaling immediately upon ligand binding. This unique feature distinguishes them from many other receptor systems that require activation.
Phosphorylation Events and Their Impact
The phosphorylation process follows a precise sequence of molecular events. Type II receptors phosphorylate type I receptors on serine and threonine residues within the GS domain. This phosphorylation event transforms inactive type I receptors into active kinases capable of downstream signaling.
Once activated, type I receptors target specific R-Smad proteins. The phosphorylation occurs at the distal C-terminal SXS motif of R-Smads. This modification is crucial because it enables R-Smad proteins to form complexes with Co-Smad proteins.
The impact of these phosphorylation events extends far beyond simple protein modification. Phosphorylated R-Smads undergo conformational changes that expose nuclear localization signals. These changes allow the protein complexes to translocate into the nucleus where they regulate gene expression.
- Conformational changes in protein structure
- Nuclear translocation of Smad complexes
- Activation of transcriptional programs
- Regulation of target gene expression
Key Kinases Involved in Phosphorylation
Several critical kinases orchestrate Smad protein phosphorylation. Type II receptors serve as the primary kinases initiating the cascade. These include TGF-β receptor II, activin receptor IIA, and bone morphogenetic protein receptor II.
Type I receptors, also known as ALK receptors, represent the secondary kinases in this system. Different ALK receptors show specificity for particular R-Smad proteins. ALK4 and ALK7 primarily phosphorylate Smad2 and Smad3, while ALK1, ALK2, ALK3, and ALK6 target Smad1, Smad5, and Smad8.
The specificity of these kinase-substrate interactions depends on structural features. L45 loop sequences within ALK receptors determine which R-Smad proteins they can phosphorylate. The phosphorylated GS motifs also contribute to this specificity by creating binding surfaces for particular R-Smads.
Pathways Associated with Smad Phosphorylation
Multiple signaling pathways converge on Smad phosphorylation mechanisms. The canonical TGF-β pathway represents the most well-characterized route. In this pathway, TGF-β ligands bind to TGF-β receptor complexes, leading to Smad2 and Smad3 phosphorylation.
The bone morphogenetic protein (BMP) pathway operates through similar mechanisms but targets different Smad proteins. BMP ligands activate ALK1, ALK2, ALK3, or ALK6 receptors, resulting in Smad1, Smad5, and Smad8 phosphorylation.
Activin and nodal signaling pathways also utilize Smad phosphorylation. These pathways share components with TGF-β signaling but can produce distinct cellular responses. The context-dependent nature of these responses highlights the importance of understanding pathway crosstalk.
Pathway integration occurs at multiple levels:
- Receptor level interactions between different complexes
- Smad protein competition for binding sites
- Transcriptional co-regulator availability
- Chromatin accessibility at target genes
We recognize that pathway crosstalk adds complexity to Smad phosphorylation analysis. Cells often receive multiple signals simultaneously, requiring sophisticated integration mechanisms. Understanding these interactions helps researchers interpret experimental results more accurately and design better therapeutic interventions.
The temporal dynamics of phosphorylation also influence pathway outcomes. Rapid phosphorylation events can produce different responses compared to sustained phosphorylation. This temporal control allows cells to distinguish between transient and persistent signals, enabling appropriate cellular responses to changing environmental conditions.
Techniques to Analyze Smad Protein Phosphorylation
The analysis of Smad protein phosphorylation demands precise methodological approaches to ensure reliable and reproducible results. We provide comprehensive guidance on selecting and implementing the most effective analytical techniques for your research objectives. Understanding the strengths and limitations of each method enables researchers to make informed decisions about their experimental design.
Modern analytical approaches offer varying levels of sensitivity, specificity, and throughput capabilities. The choice of technique depends on sample type, available resources, and the specific phosphorylation events you need to detect. We outline the most widely used methods and their practical applications in receptor-regulated SMADs research.
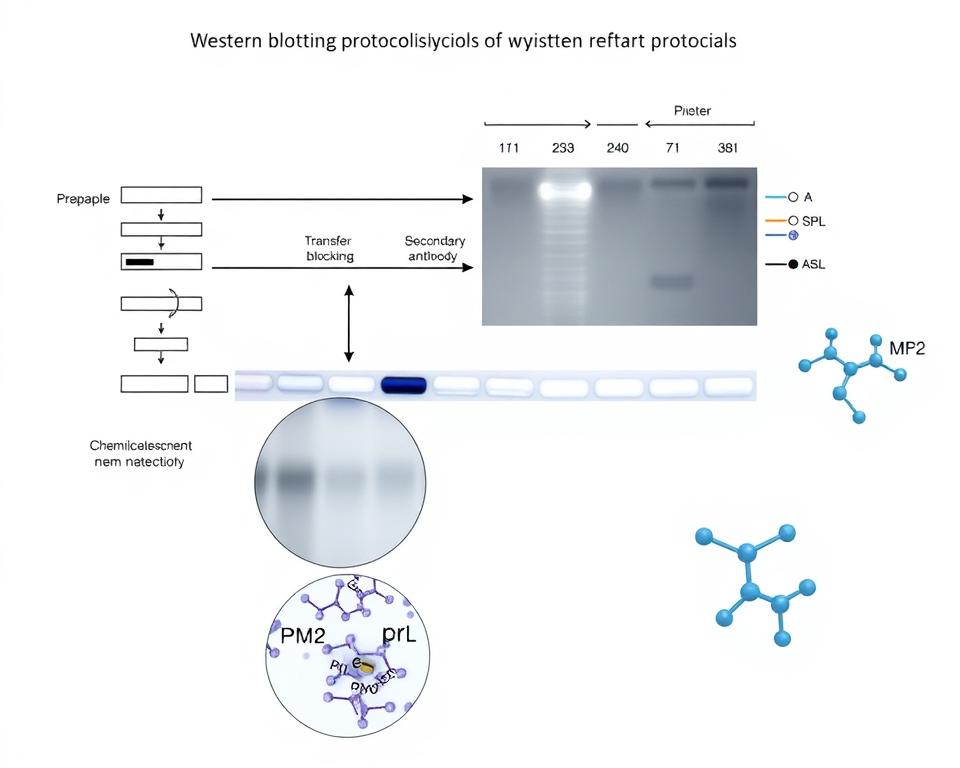
Overview of Western Blotting (WB)
Western blotting remains the gold standard for detecting and quantifying Smad protein phosphorylation. This technique provides excellent specificity through the use of phospho-specific antibodies. The method allows researchers to distinguish between total Smad proteins and their phosphorylated forms with high precision.
The fundamental principle involves protein separation by molecular weight followed by immunodetection. Phospho-specific antibodies recognize only the phosphorylated forms of target proteins. This selectivity makes Western blotting particularly valuable for studying activation states in signaling pathways.
Sample preparation plays a critical role in successful Western blot analysis. Proper lysis conditions preserve phosphorylation states while preventing protein degradation. We recommend using phosphatase inhibitors during cell lysis to maintain the integrity of phosphorylated residues.
Protocol Optimization for WB Analysis
Optimizing Western blot protocols requires careful attention to multiple variables. Antibody selection represents the most critical factor in achieving reliable results. Primary antibodies must demonstrate high specificity for the target phosphorylation site without cross-reactivity.
Loading control selection ensures accurate normalization of results. Total Smad antibodies serve as excellent loading controls when analyzing phospho-Smad levels. This approach accounts for variations in protein loading and transfer efficiency.
Detection systems vary in sensitivity and dynamic range. Chemiluminescent detection offers superior sensitivity for low-abundance phosphoproteins. Fluorescent detection provides better quantitative accuracy and allows for multiplexing capabilities.
Blocking conditions prevent non-specific antibody binding. We recommend using 5% bovine serum albumin in phosphate-buffered saline for phospho-specific antibodies. This blocking solution maintains antibody specificity while reducing background signals.
Alternative Techniques for Phosphorylation Analysis
Immunoprecipitation methods enable the detection of protein-protein interactions involving phosphorylated Smads. This technique proves particularly useful for studying receptor-Smad complexes and co-activator recruitment. The method requires careful optimization of lysis conditions and antibody concentrations.
Flow cytometry-based approaches allow single-cell analysis of phosphorylation events. This technique provides valuable information about cell population heterogeneity in signaling responses. Intracellular staining protocols must be optimized for each phospho-specific antibody.
Reverse phase protein arrays offer high-throughput analysis capabilities. This method enables simultaneous analysis of multiple phosphorylation sites across numerous samples. The technique requires specialized equipment but provides excellent quantitative data.
| Technique | Sensitivity | Throughput | Quantitative Accuracy | Sample Requirements |
|---|---|---|---|---|
| Western Blotting | High | Medium | Good | Moderate |
| Immunoprecipitation | Very High | Low | Excellent | High |
| Flow Cytometry | Medium | High | Good | Low |
| Protein Arrays | Medium | Very High | Excellent | Very High |
Mass spectrometry-based methods provide the most comprehensive phosphorylation analysis. These techniques identify specific phosphorylation sites and quantify modification levels. However, they require specialized expertise and expensive instrumentation.
Method selection should consider experimental objectives, available resources, and required sensitivity levels. Combining multiple techniques often provides the most complete understanding of Smad phosphorylation dynamics. We recommend starting with Western blotting for initial characterization before employing more specialized methods.
Applications of Studying Smad Phosphorylation
Research applications utilizing SMAD2/3 phosphorylation studies span diverse fields of biomedical science. We observe how these investigations provide critical insights into disease mechanisms and therapeutic targets. The versatility of phosphorylation analysis makes it an essential tool for understanding complex biological processes.
Scientists across multiple disciplines rely on Smad phosphorylation studies to uncover fundamental cellular mechanisms. These applications demonstrate the broad impact of TGF-β signaling research. You will find that each field offers unique perspectives on how phosphorylation events influence biological outcomes.
Implications in Cancer Research
Cancer research benefits significantly from SMAD2/3 phosphorylation analysis in tumor progression studies. We examine how altered phosphorylation patterns correlate with metastatic potential and treatment resistance. Researchers use these findings to identify novel therapeutic targets within the TGF-β pathway.
Tumor suppressor functions of Smad proteins become evident through phosphorylation studies. Dysregulated signaling often indicates cancer progression and poor patient prognosis. These investigations reveal how cancer cells manipulate normal phosphorylation events to promote growth and invasion.
Clinical applications include biomarker development for cancer diagnosis and treatment monitoring. We observe distinct phosphorylation signatures in different cancer types. This knowledge helps oncologists develop personalized treatment strategies based on individual tumor characteristics.
Role in Developmental Biology
Developmental biology research relies heavily on Smad phosphorylation analysis to understand embryonic development. We study how these signaling events regulate tissue morphogenesis and organ formation. Researchers track phosphorylation changes throughout different developmental stages.
Embryonic stem cell differentiation depends on precise SMAD2/3 phosphorylation timing and intensity. These studies reveal how cells respond to developmental cues and commit to specific lineages. Temporal regulation of phosphorylation events determines proper tissue formation.
Regenerative medicine applications emerge from understanding developmental phosphorylation patterns. We apply this knowledge to enhance tissue engineering and stem cell therapy approaches. These insights guide strategies for promoting tissue repair and regeneration.
Understanding Fibrosis and Inflammatory Diseases
Fibrosis research demonstrates how dysregulated Smad phosphorylation contributes to excessive collagen deposition. We investigate the molecular mechanisms underlying tissue scarring and organ dysfunction. These studies reveal therapeutic opportunities for treating fibrotic diseases.
Inflammatory disease research benefits from examining Smad phosphorylation in immune cell regulation. Chronic inflammation often involves altered TGF-β signaling and abnormal phosphorylation patterns. Researchers use this information to develop anti-inflammatory therapeutic strategies.
Clinical translation of fibrosis research focuses on identifying phosphorylation biomarkers for disease progression. We monitor treatment responses by tracking changes in Smad signaling activity. This approach enables early intervention and improved patient outcomes in fibrotic conditions.
Case Studies Involving Restricted Smad Phosphorylation
Comprehensive case studies illustrate the practical value of Smad phosphorylation analysis in advancing medical research. We examine three critical areas where restricted Smad phosphorylation studies have provided breakthrough insights. These investigations demonstrate how BMP signaling pathways intersect with disease mechanisms across multiple organ systems.
Research teams worldwide have documented significant findings through systematic Western blot analysis. You can observe how these studies translate laboratory discoveries into clinical applications. The following case studies represent landmark investigations that shaped our understanding of Smad protein regulation.
Examination of TGF-β Signaling in Liver Disease
Hepatocellular carcinoma research reveals complex interactions between TGF-β ligands and Smad proteins. Studies demonstrate that TGF-β can induce Smad1 phosphorylation in hepatocellular carcinoma cells. This finding challenges traditional understanding of pathway specificity.
Research teams analyzed liver tissue samples from patients with varying disease stages. Western blot analysis showed unique phosphorylation patterns in cancerous tissues. The data revealed cross-talk between TGF-β ligands and BMP signaling pathways.
Non-endothelial cell lineages within liver tissue exhibit distinct phosphorylation responses. You can observe how canonical and non-canonical pathways operate simultaneously. These findings suggest new therapeutic targets for liver cancer treatment.
Clinical implications include potential biomarker development for early detection. Researchers identified specific phosphorylation signatures associated with tumor progression. These patterns may guide personalized treatment strategies for liver cancer patients.
Smad Phosphorylation in Cardiovascular Research
Cardiovascular studies focus on endothelial cell responses to mechanical stress and growth factors. Flow-dependent Smad phosphorylation patterns emerge as critical regulators of vascular health. We observe how blood flow dynamics influence BMP signaling in arterial walls.
Endothelial cells demonstrate rapid phosphorylation changes under different flow conditions. Laminar flow promotes protective Smad phosphorylation patterns. Turbulent flow triggers inflammatory signaling cascades through altered Smad activation.
Research teams studied atherosclerosis development using advanced imaging techniques. Western blot analysis revealed distinct phosphorylation profiles in diseased versus healthy vessels. These findings connect mechanical forces to molecular signaling events.
| Flow Condition | Smad2/3 Phosphorylation | Smad1/5/8 Phosphorylation | Clinical Outcome |
|---|---|---|---|
| Laminar Flow | Moderate Increase | Significant Increase | Vascular Protection |
| Turbulent Flow | High Increase | Decreased Activity | Atherosclerosis Risk |
| Static Conditions | Baseline Levels | Minimal Activity | Endothelial Dysfunction |
| Oscillatory Flow | Variable Response | Irregular Patterns | Inflammation Markers |
Therapeutic interventions targeting flow-sensitive pathways show promise in clinical trials. You can apply these findings to develop treatments for cardiovascular disease. The research demonstrates how BMP signaling modulation affects vascular remodeling processes.
Investigating Neurodegenerative Disorders
Neurodegenerative disease research reveals altered Smad signaling in neuronal dysfunction. Studies examine how phosphorylation changes contribute to disease progression. We investigate connections between BMP signaling disruption and cognitive decline.
Alzheimer’s disease models show reduced Smad phosphorylation in affected brain regions. Researchers analyzed post-mortem tissue samples using quantitative Western blotting. The data indicates progressive loss of normal signaling patterns.
Parkinson’s disease studies demonstrate different phosphorylation abnormalities. Dopaminergic neurons exhibit specific Smad activation defects. These changes precede visible symptoms by months or years.
Amyotrophic lateral sclerosis research identifies motor neuron-specific phosphorylation patterns. Studies show how BMP signaling alterations affect neuronal survival. The findings suggest potential intervention points for treatment development.
Clinical translation efforts focus on biomarker development for early detection. Cerebrospinal fluid analysis reveals measurable phosphorylation changes. You can use these markers to monitor disease progression and treatment response.
Therapeutic strategies target upstream regulators of Smad phosphorylation. Drug development programs explore BMP signaling modulators for neuroprotection. Early clinical trials show encouraging results for slowing disease progression.
These case studies demonstrate the practical value of Western blot analysis in translating basic research findings. We observe how systematic investigation of Smad phosphorylation advances clinical understanding. The research provides foundation for developing targeted therapeutic interventions across multiple disease areas.
Comparing Smad Phosphorylation Across Species
Comparative studies across different organisms illuminate the evolutionary significance of Smad phosphorylation mechanisms. We observe remarkable conservation of these signaling pathways from simple invertebrates to complex mammals. This cross-species analysis reveals fundamental principles that govern cellular communication through phosphorylated SMAD complexes.
The evolutionary preservation of Smad proteins demonstrates their critical importance in cellular function. You can trace these signaling molecules across millions of years of evolution. Their conservation suggests that phosphorylated SMAD complexes perform essential roles that cannot be easily replaced by alternative mechanisms.
Evolutionary Perspectives on Smad Proteins
Smad proteins originated early in metazoan evolution and maintained their core structure across species. The Drosophila homolog MAD (Mothers Against Decapentaplegic) shares significant sequence similarity with mammalian Smads. This conservation extends to the phosphorylation sites that regulate protein activity.
Caenorhabditis elegans contains SMA proteins that function similarly to vertebrate Smads. These nematode proteins undergo comparable phosphorylation events. The preservation of these mechanisms across such diverse organisms highlights the fundamental importance of phosphorylated SMAD complexes in cellular signaling.

WB visualization of Smad2 (Ser250) phosphorylation
Structural analysis reveals that key phosphorylation domains remain highly conserved. The C-terminal SSXS motif appears in Smad proteins across multiple phyla. This conservation suggests that the basic mechanism of Smad activation through phosphorylation emerged early and proved highly successful.
Species Variation in Signaling Pathways
Despite overall conservation, we observe interesting variations in Smad signaling between species. Different organisms show distinct patterns of receptor specificity and downstream target genes. These variations reflect adaptations to specific physiological needs and environmental pressures.
Mammalian systems typically contain more Smad protein isoforms than invertebrate models. This expansion allows for greater signaling complexity and tissue-specific responses. However, the core phosphorylation mechanisms that activate these proteins remain remarkably similar across species.
Fish and amphibian models demonstrate unique aspects of Smad signaling during development. Their phosphorylated SMAD complexes regulate processes like regeneration that are limited in mammals. These differences provide valuable insights into the evolutionary potential of Smad pathways.
Plant organisms lack true Smad proteins but contain functionally similar signaling molecules. This parallel evolution suggests that phosphorylation-based signal transduction represents an optimal solution for cellular communication. The convergent evolution supports the importance of these mechanisms.
Implications for Biomedical Research
Cross-species conservation of Smad phosphorylation validates the use of model organisms in research. Studies in Drosophila and C. elegans provide relevant insights into human disease mechanisms. The conservation of phosphorylated SMAD complexes ensures that findings translate effectively between species.
Mouse models remain the gold standard for studying mammalian Smad signaling. Their close evolutionary relationship to humans means that phosphorylated SMAD complexes function nearly identically. This similarity makes mouse studies highly predictive of human responses.
Zebrafish offer unique advantages for studying Smad signaling during development. Their transparent embryos allow real-time visualization of signaling events. You can observe how phosphorylated SMAD complexes regulate tissue formation and organ development.
Understanding species differences helps researchers choose appropriate model systems. Some aspects of Smad signaling are best studied in specific organisms. For example, regeneration studies benefit from salamander models, while cancer research often relies on mouse systems.
The evolutionary perspective also identifies potential therapeutic targets. Conserved phosphorylation sites represent promising intervention points. Drugs targeting these sites are more likely to be effective across different patient populations due to the underlying evolutionary conservation.
Selection of Appropriate Controls in Experiments
Experimental controls serve as the cornerstone for accurate interpretation of R-SMAD activation patterns in research studies. We understand that proper control selection directly impacts the validity and reproducibility of your western blotting results. Without appropriate controls, distinguishing genuine phosphorylation signals from experimental artifacts becomes nearly impossible.
The complexity of Smad protein signaling pathways demands rigorous experimental design. You must implement multiple control types to validate each aspect of your phosphorylation analysis. This systematic approach ensures that your research conclusions accurately reflect biological processes rather than technical variations.
Importance of Control Samples in WB
Control samples provide the reference points necessary for meaningful data interpretation in western blotting experiments. We recommend implementing controls that address both technical and biological variables in your phosphorylation studies. These controls help you identify potential sources of error and validate the specificity of your results.
Negative controls using unstimulated cells establish baseline phosphorylation levels. These samples demonstrate the basal state of Smad proteins before activation occurs. You can compare treated samples against these controls to quantify the magnitude of phosphorylation changes.
Positive controls with known activators confirm that your experimental system responds appropriately to stimulation. TGF-β treatment typically serves as an effective positive control for R-SMAD activation studies. These controls verify that your cells retain the capacity for normal signaling responses.
Loading controls ensure equal protein amounts across all samples in your analysis. β-actin, GAPDH, or total Smad proteins commonly serve this purpose. Proper loading controls prevent misinterpretation of results due to uneven sample loading.
Recommended Controls for Phosphorylation Studies
We provide specific control recommendations based on extensive experience with Smad phosphorylation analysis. Your experimental design should incorporate multiple control types to address different aspects of the signaling pathway. Each control type serves a distinct validation purpose in your research.
| Control Type | Purpose | Implementation | Expected Result |
|---|---|---|---|
| Negative Control | Baseline phosphorylation | Unstimulated cells | Minimal signal |
| Positive Control | Maximum activation | TGF-β treatment | Strong phosphorylation |
| Time Course Control | Kinetic validation | Multiple time points | Progressive changes |
| Inhibitor Control | Pathway specificity | Kinase inhibitors | Reduced activation |
Time-course controls reveal the temporal dynamics of phosphorylation events. You should collect samples at multiple time points to understand the kinetics of R-SMAD activation. This approach helps identify optimal treatment durations for your specific experimental conditions.
Dose-response controls demonstrate the relationship between stimulus concentration and phosphorylation intensity. We recommend testing multiple concentrations of your treatment agent. This analysis reveals the sensitivity of your system and helps optimize treatment conditions.
Inhibitor controls validate pathway specificity by blocking key signaling components. SB431542 effectively inhibits TGF-β receptor kinase activity and serves as an excellent specificity control. These controls confirm that observed phosphorylation depends on the intended signaling pathway.
Troubleshooting Common Control Issues
Control-related problems frequently compromise experimental results and lead to misinterpretation of data. We address the most common issues that researchers encounter during Smad phosphorylation analysis. Understanding these problems helps you implement effective solutions quickly.
Background signal in negative controls often indicates antibody cross-reactivity or inadequate blocking conditions. You can reduce background by optimizing antibody concentrations and extending blocking times. Primary antibody dilution adjustments frequently resolve this issue.
Weak positive control signals suggest problems with cell viability or treatment conditions. Check your TGF-β stock concentration and storage conditions. Ensure that cells remain healthy throughout the treatment period and maintain appropriate culture conditions.
Uneven loading controls indicate sample preparation inconsistencies or pipetting errors. We recommend using protein quantification assays before sample preparation. Bradford or BCA assays provide accurate protein concentration measurements for consistent loading.
Sample degradation affects both experimental and control samples but may appear more pronounced in controls. Store samples at appropriate temperatures and include protease inhibitors in your lysis buffer. Process samples quickly to minimize protein degradation during preparation.
Antibody specificity issues can cause unexpected signals in control samples. Validate your antibodies using knockout cell lines or specific inhibitors when possible. This verification ensures that detected signals represent genuine phosphorylation events rather than cross-reactive binding.
Challenges in Analyzing Smad Phosphorylation
Research teams encounter significant obstacles when studying SMAD nuclear translocation and phosphorylation patterns. These challenges stem from the dynamic nature of cellular signaling and the technical limitations of current analytical methods. We recognize that overcoming these hurdles requires systematic approaches and careful experimental design.
The transient nature of phosphorylation events creates timing-sensitive detection windows. Researchers must capture these brief molecular interactions before they disappear. This temporal challenge becomes even more complex when studying cell-type specific responses that vary across different tissue environments.
Variability in Protein Expression Levels
Protein expression levels fluctuate dramatically between cell types and experimental conditions. This variability affects detection sensitivity and requires robust normalization strategies. We observe that baseline Smad protein levels can differ by several fold between samples.
Cell culture conditions significantly impact protein expression patterns. Serum concentrations, growth factors, and passage numbers all influence Smad protein abundance. These factors create background noise that complicates phosphorylation analysis.
Standardizing sample preparation becomes critical for reliable results. Loading controls must account for both total protein content and specific Smad expression levels. This dual normalization approach helps minimize experimental variability.
Issues with Antibody Specificity and Sensitivity
Antibody cross-reactivity presents major challenges in phosphorylation studies. Many commercial antibodies show unexpected binding to multiple Smad species or phosphorylation sites. This cross-reactivity leads to false positive signals and misinterpretation of results.
Sensitivity limitations affect detection of low-abundance phosphorylated species. Some antibodies require high protein concentrations that may not reflect physiological conditions. We recommend thorough antibody validation using multiple approaches.
Batch-to-batch variability in antibody performance creates reproducibility issues. Different lots of the same antibody can show varying specificity profiles. Researchers should validate each new antibody batch before critical experiments.
Interpretative Challenges in Data Analysis
Distinguishing between canonical and non-canonical phosphorylation events requires specialized knowledge. SMAD nuclear translocation patterns can vary depending on which phosphorylation sites are modified. This complexity demands careful interpretation of Western blot results.
Temporal dynamics add another layer of analytical difficulty. Phosphorylation levels change rapidly following stimulation, creating time-dependent signal patterns. Researchers must consider these kinetics when designing experiments and interpreting data.
Statistical analysis of phosphorylation data presents unique challenges. Traditional methods may not account for the non-linear relationships between stimulus intensity and phosphorylation response. We recommend using specialized statistical approaches designed for signaling data.
Practical solutions include implementing rigorous controls, validating antibodies thoroughly, and using complementary analytical techniques. These strategies help researchers navigate the complex landscape of Smad phosphorylation analysis while maintaining data quality and reproducibility.
Future Directions in Smad Protein Research
Cutting-edge technologies are reshaping the future of inhibitory SMAD proteins research. We stand at the threshold of revolutionary discoveries that will transform our understanding of cellular signaling mechanisms. These advances promise to unlock new therapeutic possibilities and enhance our ability to combat complex diseases.
The integration of advanced analytical methods with traditional research approaches creates unprecedented opportunities. You will witness how emerging technologies accelerate the pace of discovery in Smad pathway investigations. Our field continues to evolve through innovative methodologies that provide deeper insights into protein phosphorylation dynamics.
Emerging Technologies in Protein Analysis
Single-cell analysis represents a paradigm shift in studying inhibitory SMAD proteins at unprecedented resolution. This technology enables researchers to examine phosphorylation events within individual cells. We can now observe cellular heterogeneity that was previously masked in bulk population studies.
Mass spectrometry advances continue to revolutionize protein phosphorylation analysis. High-resolution instruments detect subtle modifications with remarkable precision. You benefit from improved sensitivity that reveals previously undetectable phosphorylation sites on inhibitory SMAD proteins.
Reverse phase protein arrays offer high-throughput capabilities for phosphorylation screening. These platforms process hundreds of samples simultaneously with consistent results. We utilize automated systems that reduce experimental variability while increasing data quality.
Potential Therapeutic Targets within Smad Pathways
Cancer therapeutics increasingly focus on inhibitory SMAD proteins as intervention points. These proteins regulate tumor suppressor pathways that control cell proliferation. We identify specific phosphorylation sites that could serve as drug targets for precision medicine approaches.
Fibrosis treatment strategies target Smad signaling cascades to prevent excessive tissue scarring. Researchers develop small molecule inhibitors that modulate phosphorylation patterns. You observe promising results in preclinical models of liver, lung, and kidney fibrosis.
Inflammatory disease management benefits from understanding inhibitory SMAD proteins regulation. These pathways control immune cell activation and cytokine production. We explore therapeutic interventions that restore balanced signaling in autoimmune conditions.
Cardiovascular applications target Smad phosphorylation in vascular remodeling processes. Novel therapeutic approaches prevent pathological changes in blood vessel structure. Research demonstrates potential for treating hypertension and atherosclerosis through Smad pathway modulation.
| Disease Category | Target Pathway | Therapeutic Approach | Development Stage |
|---|---|---|---|
| Cancer | Inhibitory SMAD proteins | Small molecule inhibitors | Phase II trials |
| Fibrosis | TGF-β/Smad signaling | Phosphorylation modulators | Preclinical |
| Inflammatory diseases | Smad regulatory networks | Pathway restoration | Early development |
| Cardiovascular | Vascular Smad signaling | Targeted interventions | Research phase |
The Role of Bioinformatics in Smad Research
Computational biology transforms how we analyze inhibitory SMAD proteins phosphorylation networks. Machine learning algorithms identify complex patterns in large datasets. We leverage artificial intelligence to predict phosphorylation sites and their functional consequences.
Systems biology approaches integrate multiple data types to create comprehensive pathway models. These models predict how perturbations affect entire signaling networks. You access sophisticated tools that simulate cellular responses to therapeutic interventions.
Database integration enables researchers to combine experimental results with existing knowledge. Standardized formats facilitate data sharing across research institutions. We contribute to collaborative efforts that accelerate scientific discovery through open data initiatives.
Predictive modeling helps prioritize experimental approaches and therapeutic targets. Advanced algorithms forecast which inhibitory SMAD proteins modifications will have the greatest impact. Research teams optimize their efforts by focusing on the most promising avenues identified through computational analysis.
The convergence of experimental and computational approaches creates synergistic effects in Smad research. We anticipate continued growth in bioinformatics applications that enhance our understanding of protein phosphorylation mechanisms. These technological advances position us to make breakthrough discoveries that translate into improved patient outcomes.
Regulatory Mechanisms of Smad Phosphorylation
Regulatory mechanisms controlling Smad phosphorylation extend far beyond simple receptor activation events. We observe that restricted smad protein phosphorylation operates through sophisticated control systems that ensure precise cellular responses. These mechanisms involve multiple layers of molecular regulation that fine-tune signaling outcomes.
The complexity of Smad regulation becomes apparent when examining the diverse modification patterns. Multiple kinases and phosphatases work together to control phosphorylation states. This intricate network prevents excessive pathway activation while maintaining cellular responsiveness.
Post-Translational Modifications
Post-translational modifications represent the primary control mechanism for Smad protein activity. Linker region phosphorylation by MAPK pathways creates distinct regulatory patterns that differ from canonical C-terminal phosphorylation. These modifications directly influence nuclear translocation and transcriptional activity.
Ubiquitination serves as another critical modification affecting Smad stability and localization. E3 ligases target specific Smad proteins for degradation or nuclear export. This process helps terminate signaling responses and prevents prolonged pathway activation.
Acetylation events add another layer of complexity to Smad regulation. Histone acetyltransferases and deacetylases modify Smad proteins to control their DNA-binding capacity. These modifications work alongside phosphorylation to create combinatorial regulatory codes.
Feedback Mechanisms in Signaling Pathways
Feedback loops maintain homeostasis within the SMAD signaling pathway through multiple regulatory circuits. Negative feedback mechanisms involve inhibitory Smads that compete with receptor-regulated Smads for binding sites. This competition prevents signal amplification beyond physiological limits.
Positive feedback loops can amplify initial signals under specific conditions. Certain Smad target genes encode proteins that enhance pathway sensitivity. These mechanisms create bistable switches that commit cells to particular developmental fates.
Temporal control represents another feedback dimension affecting restricted smad protein phosphorylation. Oscillatory patterns emerge from delayed negative feedback loops. These dynamics enable cells to distinguish between transient and sustained stimuli.
Crosstalk with Other Signaling Molecules
Extensive crosstalk connects Smad pathways with other major signaling networks. MAPK pathway interactions create integration points where multiple signals converge. ERK and p38 kinases phosphorylate Smad linker regions to modulate their activity.
PI3K/Akt signaling influences Smad function through direct phosphorylation events. This crosstalk enables growth factor signals to modify TGF-β responses. The integration creates context-dependent cellular outcomes.
Wnt pathway components interact with Smad proteins at multiple levels. β-catenin can form complexes with Smads to influence target gene selection. These interactions demonstrate how the SMAD signaling pathway integrates diverse developmental cues.
We recognize that understanding these regulatory mechanisms provides crucial insights for therapeutic intervention. Each control point represents a potential target for modulating pathway activity in disease contexts.
Insights into the Clinical Relevance of Smad Phosphorylation
Understanding TGF-beta induced phosphorylation patterns offers unprecedented opportunities for precision medicine. Clinical research demonstrates that Smad phosphorylation analysis provides valuable insights for disease diagnosis, treatment selection, and patient monitoring. We observe significant correlations between phosphorylation profiles and clinical outcomes across multiple disease states.
The translation of basic research findings into clinical applications represents a critical advancement in modern healthcare. Receptor-regulated SMADs serve as reliable indicators of cellular signaling activity in various pathological conditions. These molecular markers enable clinicians to make more informed treatment decisions based on individual patient profiles.
Biomarkers for Disease Prediction
Clinical studies reveal that TGF-beta induced phosphorylation patterns function as powerful predictive biomarkers. We identify specific phosphorylation signatures that correlate with disease progression in cancer, fibrosis, and inflammatory conditions. These biomarkers enable early detection and risk stratification in patient populations.
Diagnostic applications focus on measuring phosphorylated Smad levels in tissue samples and blood specimens. The analysis provides quantitative data that supports clinical decision-making processes. Healthcare providers use these measurements to assess disease severity and monitor treatment effectiveness over time.
Prognostic value emerges from longitudinal studies tracking phosphorylation changes. Patients with altered receptor-regulated SMADs activity show distinct clinical trajectories. This information helps predict treatment outcomes and guides therapeutic planning strategies.
Therapeutic Interventions Targeting Smad Signaling
Targeted therapies focus on modulating Smad phosphorylation pathways through various mechanisms. Small molecule inhibitors block specific kinases responsible for phosphorylation events. These compounds show promise in treating diseases characterized by excessive TGF-beta signaling activity.
Receptor antagonists represent another therapeutic approach for controlling Smad activation. We develop strategies that prevent inappropriate phosphorylation while maintaining normal cellular functions. Clinical trials evaluate the safety and efficacy of these targeted interventions.
Combination therapies integrate Smad pathway modulators with conventional treatments. This approach enhances therapeutic effectiveness while reducing potential side effects. The synergistic effects improve patient outcomes across diverse disease conditions.
Patient Response to Treatments Involving Smad Protein Pathways
Personalized medicine approaches utilize receptor-regulated SMADs profiles to predict treatment responses. We analyze individual phosphorylation patterns to identify patients likely to benefit from specific therapies. This strategy optimizes treatment selection and improves clinical outcomes.
Monitoring protocols track changes in Smad phosphorylation during treatment courses. Regular assessment enables real-time adjustments to therapeutic regimens. Healthcare teams use this data to optimize dosing schedules and treatment duration.
Response biomarkers help distinguish between treatment responders and non-responders early in therapy. Patients showing favorable phosphorylation changes demonstrate better long-term outcomes. This information guides continuation or modification of treatment protocols for individual patients.
Conclusion: The Significance of Restricted Smad Protein Phosphorylation
The comprehensive analysis of restricted Smad protein phosphorylation reveals fundamental mechanisms that drive cellular communication and disease pathogenesis. We have explored how these critical signaling molecules regulate diverse biological processes through precise molecular interactions.
Summary of Key Findings
Research demonstrates that SMAD2/3 phosphorylation serves as a central regulatory mechanism in multiple cellular pathways. The studies examined show how phosphorylated SMAD complexes function as essential mediators between extracellular signals and nuclear transcriptional responses. BMP signaling pathways particularly highlight the intricate balance required for proper cellular function.
The Future of Smad Research in Health and Disease
Emerging technologies offer unprecedented opportunities to understand complex regulatory networks governing Smad protein function. Advanced analytical techniques will enable researchers to map detailed phosphorylation patterns and their clinical implications. These developments promise to unlock new therapeutic targets for treating various diseases.
Call to Further Investigate Smad Pathways
We encourage continued exploration of Smad signaling mechanisms to advance biomedical research. The potential for developing innovative therapeutic strategies remains substantial. Future investigations should focus on translating laboratory findings into clinical applications that benefit patient outcomes. Understanding these pathways will drive the next generation of targeted treatments and diagnostic tools.
References and further readings:
1.Luo K. Signaling Cross Talk between TGF-β/Smad and Other Signaling Pathways. Cold Spring Harb Perspect Biol. 2017;9(2):a022137. doi:10.1101/cshperspect.a022137. PMID:27836834
2.van Grunsven LA, Schellens A, Huylebroeck D, Verschueren K. SIP1 (Smad Interacting Protein 1) and deltaEF1 (delta-Crystallin Enhancer Binding Factor) Are Structurally Similar Transcriptional Repressors. J Bone Joint Surg Am. 2001;83-A(4):594-602. doi:10.2106/00004623-200104000-00014. PMID:11263664
3.Zwijsen A, Verschueren K, Huylebroeck D. New Intracellular Components of Bone Morphogenetic Protein/Smad Signaling Cascades. FEBS Lett. 2003;546(1):1-7. doi:10.1016/S0014-5793(03)00566-0. PMID:128292494.Miyazono K. Signal Transduction by Bone Morphogenetic Protein Receptors: Functional Roles of Smad Proteins. Bone. 1999;25(1 Suppl):1S-6S. doi:10.1016/S8756-3282(99)00113-1. PMID:10423029
5.Bruce DL, Sapkota GP. Phosphatases in SMAD Regulation. FEBS Lett. 2012;586(6):763-772. doi:10.1016/j.febslet.2012.02.001. PMID:22576046
FAQ
What are restricted Smad proteins and why is their phosphorylation important?
Restricted Smad proteins are receptor-regulated SMADs (R-SMADs) that include SMAD1, SMAD2, SMAD3, SMAD5, and SMAD8. These evolutionarily conserved transcription factors become phosphorylated by specific type I receptors in response to TGF-beta induced phosphorylation and BMP signaling. Their phosphorylation is crucial because it enables SMAD nuclear translocation and formation of phosphorylated SMAD complexes that regulate gene expression in developmental processes and disease pathogenesis.
How does the SMAD signaling pathway work at the molecular level?
The SMAD signaling pathway begins when transforming growth factor-β superfamily members bind to heteromeric receptor complexes comprising two type II and two type I receptors. Constitutively active type II receptors phosphorylate type I receptors in their GS domains, which subsequently leads to receptor-regulated SMADs phosphorylation. This R-SMAD activation enables complex formation with Co-SMADs and nuclear translocation to regulate target gene transcription.
What is the difference between R-SMADs, Co-SMADs, and I-SMADs?
Receptor-regulated SMADs (R-SMADs) like SMAD2 and SMAD3 are directly phosphorylated by type I receptors. Co-SMADs such as SMAD4 form complexes with activated R-SMADs for nuclear translocation. Inhibitory SMAD proteins (I-SMADs) including SMAD6 and SMAD7 act as negative regulators by preventing R-SMAD phosphorylation and complex formation, maintaining signaling homeostasis within the pathway.
What makes Western blotting effective for detecting SMAD2/3 phosphorylation?
Western blotting provides precise detection of SMAD2/3 phosphorylation through specific antibodies that distinguish between total SMAD proteins and their phosphorylated forms. We optimize protocols including proper sample preparation, antibody selection, and detection systems to ensure reliable quantification of activation states. The technique allows accurate measurement of phosphorylation levels across different experimental conditions and time points.
How does BMP signaling differ from TGF-β signaling in SMAD phosphorylation?
BMP signaling primarily activates SMAD1, SMAD5, and SMAD8 through ALK1, ALK2, ALK3, and ALK6 receptors, while TGF-β signaling activates SMAD2 and SMAD3 through ALK4, ALK5, and ALK7 receptors. The specificity depends on L45 loop sequences and phosphorylated GS motifs in different ALK receptors, determining precise signaling outcomes and downstream gene expression patterns in various cellular contexts.
What alternative techniques can complement Western blotting for phosphorylation analysis?
We utilize immunoprecipitation, flow cytometry, and reverse phase protein arrays as complementary techniques for comprehensive phosphorylation analysis. These methods provide different advantages such as protein-protein interaction studies, single-cell analysis capabilities, and high-throughput screening. The selection depends on experimental objectives, sample types, and required sensitivity levels for detecting phosphorylated SMAD complexes.
What are the main applications of SMAD phosphorylation studies in disease research?
SMAD phosphorylation analysis provides crucial insights into cancer research by revealing altered TGF-β signaling in tumor progression and metastasis. We apply these studies in developmental biology to understand embryonic development regulation, fibrosis research to investigate excessive collagen deposition, and cardiovascular disease to examine endothelial cell responses. These applications demonstrate the versatility across multiple research disciplines.
What controls are essential for reliable SMAD phosphorylation analysis?
We recommend negative controls using unstimulated cells, positive controls with known activators like TGF-β1, and loading controls for protein normalization. Essential control strategies include time-course controls, dose-response controls, and inhibitor controls using compounds like SB431542 to validate R-SMAD activation specificity. These controls ensure experimental reproducibility and accurate data interpretation.
What are the main challenges in analyzing SMAD nuclear translocation?
Key challenges include protein expression variability affecting detection sensitivity, antibody specificity issues causing cross-reactivity between different SMAD species, and distinguishing between canonical and non-canonical phosphorylation events. We address these through improved experimental design, careful normalization strategies, validation approaches, and accounting for temporal dynamics and cell-type specific responses in SMAD nuclear translocation studies.
How do inhibitory SMAD proteins regulate the signaling pathway?
Inhibitory SMAD proteins like SMAD6 and SMAD7 prevent excessive pathway activation by competing with R-SMADs for receptor binding, promoting receptor degradation, and recruiting phosphatases. They maintain signaling homeostasis through feedback mechanisms and provide critical regulation points. These proteins are often upregulated by TGF-β signaling itself, creating negative feedback loops that prevent sustained pathway activation.
What post-translational modifications affect SMAD protein function beyond phosphorylation?
Beyond restricted SMAD protein phosphorylation, we examine linker region phosphorylation, ubiquitination, and acetylation that fine-tune signaling activity. These modifications affect protein stability, nuclear translocation efficiency, and transcriptional activity. Crosstalk with MAPK, PI3K/Akt, and Wnt pathways provides additional regulatory layers, ensuring precise control of cellular responses and multiple therapeutic intervention points.
How can SMAD phosphorylation patterns serve as clinical biomarkers?
Receptor-regulated SMAD phosphorylation profiles correlate with disease progression, treatment response, and patient outcomes across various conditions. We demonstrate how these patterns serve as diagnostic and prognostic biomarkers, enabling personalized medicine approaches. Therapeutic interventions targeting SMAD pathways include small molecule inhibitors and receptor antagonists, with phosphorylation biomarkers helping predict treatment responses and optimize therapeutic strategies.
Leo Bios
Hello, I’m Leo Bios. As an assistant lecturer, I teach cellular and
molecular biology to undergraduates at a regional US Midwest university. I started as a research tech in
a biotech startup over a decade ago, working on molecular diagnostic tools. This practical experience
fuels my teaching and writing, keeping me engaged in biology’s evolution.





Leave a Comment
Your email address will not be published. Required fields are marked *